Medically reviewed by Dr. Jordan Pinsker, Chief Medical Officer, Tandem Diabetes Care
Learning how to use a continuous glucose monitoring sensor, also called a continuous glucose monitor or a CGM sensor, is an essential part of diabetes management — regardless of whether someone is living with type 1 diabetes or type 2 diabetes. This article will explain how a CGM sensor works, how it helps to monitor blood glucose levels, and how people who combine a CGM with automated insulin delivery (AID) have the best overall glucose control compared to multiple daily injections or standard pump therapy.1
Understanding Continuous Glucose Monitoring
A continuous glucose monitoring sensor is a small medical device that provides real-time blood sugar readings that can help someone living with diabetes see when their glucose levels are either rising or falling. This is also called “going high” or “going low.”
The technology, which was developed in the late 1990s, has drastically evolved to help people minimize the amount of times they need to use fingersticks and a glucose meter to prick their finger to test their blood.
Using a CGM sensor does not completely eliminate the need for blood glucose tests — which could be fingersticks or a multi-piece glucose meter system consisting of test strips, a lancet, the meter, alcohol swabs, etc. But it can reduce the frequency that these tests are needed because a CGM is constantly monitoring blood sugars.
Using a continuous glucose monitor also opens up the opportunity for someone living with diabetes to use an automated insulin delivery system — which is when an insulin pump with a predictive algorithm uses the CGM sensor readings to automatically adjust insulin dosing.
"A CGM is a tool that has allowed healthcare providers insight into the dynamic environment of glycemic changes in patients who have diabetes,” said Amy Rich, DNP, CRNP, CDCES, and a Medical Science Liaison with Tandem Diabetes Care. “This tool allows for better individualized care plans that can promote better outcomes and results for patients."
Added Leah Fuller, Senior Medical Science Liaison Manager for Tandem Diabetes Care: “It would take hundreds of fingersticks a day to get the same amount of information available when wearing a CGM. That information is invaluable for people with diabetes when they are making decisions throughout the day. But making hundreds of therapy decisions a day is burdensome for people with diabetes. Being able to send the data from the CGM directly to an insulin pump takes some of that burden off the person without compromising safety and outcomes.”
How do Continuous Glucose Monitoring Sensors Work?
Continuous glucose monitoring works by measuring the glucose levels in the fluid just beneath the skin, called interstitial fluid.
Most CGMs are attached to the skin by using a microneedle and spring-loaded applicator. Because a continuous glucose monitoring sensor can be worn on-body for several days, it minimizes the need for someone to prick their finger to draw blood to test with a glucose meter several times a day.
Common sites for wearing a CGM sensor include the back of the arm, the abdomen, or the upper buttocks — depending on the brand and model of the CGM sensor. (Note: Always consult with a healthcare provider about what areas on the body are appropriate for using a continuous glucose monitoring sensor. It may change depending on the brand.)
Those sensor readings are then sent via Bluetooth® to either a receiver or a mobile app on a compatible smartphone so someone can discreetly monitor their glucose levels.
Some insulin pumps also have a touchscreen interface that allows to users to view their CGM glucose levels directly on their insulin pump.

Advantages of Continuous Glucose Monitoring Sensors
As the accuracy of CGM sensors improved over the years, they eventually became an essential tool in reducing the burden of diabetes management. The American Diabetes Association (ADA) began recommending continuous glucose monitoring for people living with type 1 diabetes in 2018.
Continuous glucose monitoring is now being used by many people with diabetes — including those with type 2 diabetes. The ability to measure glucose levels in real time provides numerous advantages for anyone who is taking medications to treat diabetes.
For example, someone could see that their glucose levels are trending in a certain direction and act quickly to correct the problem without the need to use a fingerstick every few minutes. If someone's glucose is predicted to go low, a CGM would alert them. They could have a "low snack" on hand to raise their glucose before it is below the safe range. Also, they wouldn't have to wait until they were feeling symptoms of hypoglycemia that would prompt them to use a fingerstick.
Conversely, if a person sees their glucose levels are starting to rise, they could take several actions. For example, if high blood sugars are occurring after meals, this could mean a walk or some exercise after dinner might be helpful. Or, a person could have insulin on hand or give themself insulin from their insulin pump.
A CGM sensor is also a valuable tool for people living with diabetes and their healthcare providers to track specific trends and gain insights into how certain foods or activities can impact glucose levels.
Having real-time data on how certain foods impact blood sugar could be extremely helpful because food intake plays such an important role in diabetes management.
It's also helpful to review type 1 diabetes food plans and food lists. Having a baseline understanding of the glycemic index, along with real-time glucose management, could help improve time in range and could also help lower A1c.
“CGMs allow users to see the direct impact of food choices, exercise, medication, and many other factors of glucose control,” Leah Fuller said. “That is incredibly helpful, as identifying these patterns and adjusting habits can have a large impact on keeping glucose levels in a safe range.”
Integration with Automated Insulin Delivery Systems
When a CGM is integrated with an insulin pump that has a predictive algorithm, it creates what’s called an automated insulin delivery system.
The predictive algorithm uses the continuous glucose monitoring sensor readings to predict where glucose levels may be in the near future, and then automatically adjusts insulin dosing by either reducing or suspending insulin delivery if glucose is predicted to go low, or increasing insulin delivery if glucose is predicted to rise.
AID systems have been shown to reduce the burden of diabetes management because of automation, which leads to less mental burden thanks to having to make fewer decisions.
The ADA recommends offering automated insulin delivery systems to all people with type 1 diabetes who are capable of using the devices safely.2
“Automated insulin delivery systems have been proven to improve outcomes in people of all ages with type 1 diabetes,” said Dr. Jordan Pinsker, Chief Medical Officer at Tandem Diabetes Care. “By combining an insulin pump, a CGM device, and a computerized algorithm to connect the two, we consistently see reduced burden of diabetes management and vastly improved outcomes.”
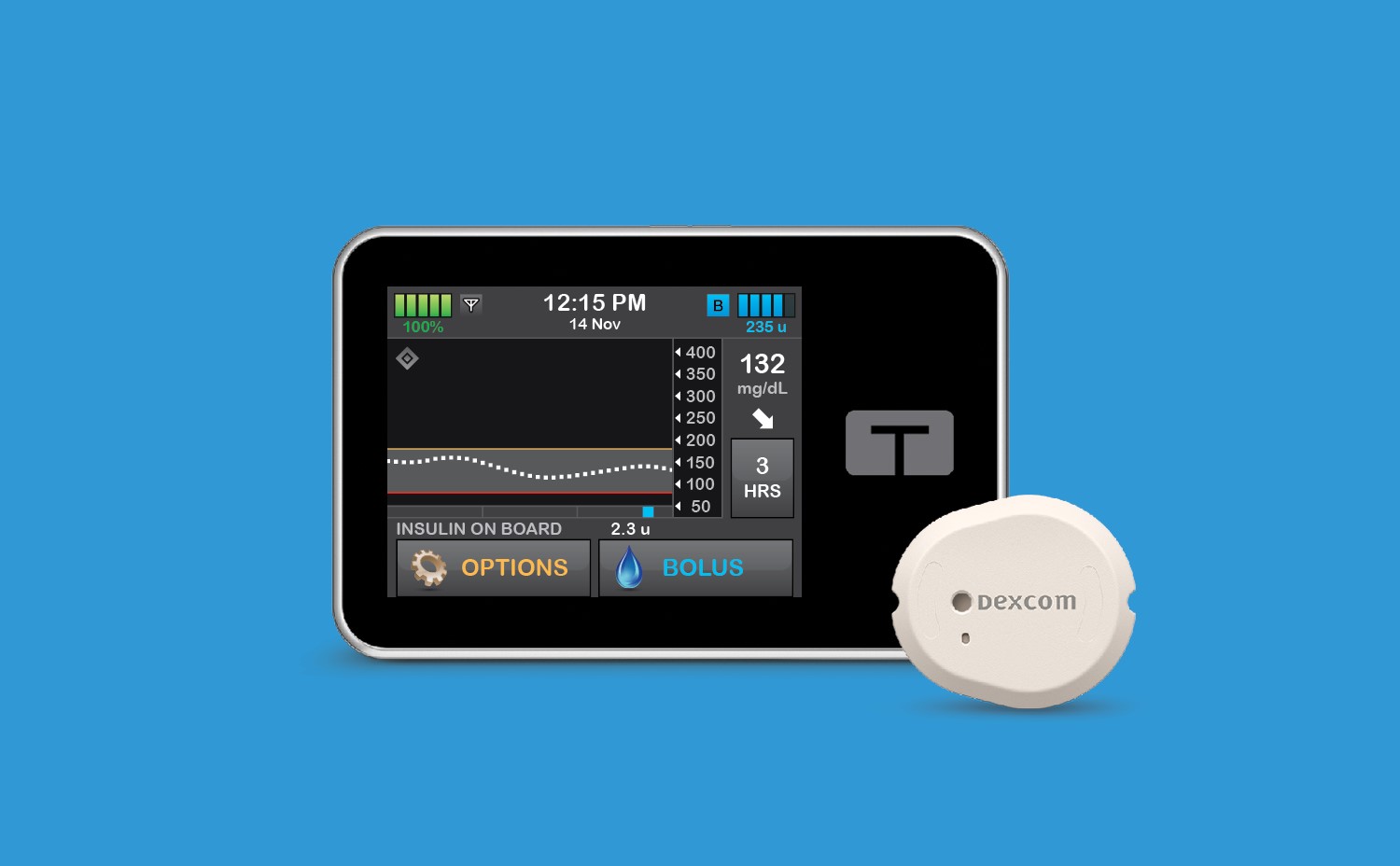
Tandem Diabetes Care CGM integration
Tandem Diabetes Care, which makes the Tandem Mobi system and t:slim X2 insulin pump, is the first company to have multiple automated insulin delivery systems that are compatible with multiple CGM sensors.
Both pumps are powered by Control-IQ, an advanced hybrid closed-loop technology, that predicts and helps prevent highs and lows.
The Tandem Mobi system is compatible with the Dexcom G7 continuous glucose monitoring sensor and the Dexcom G6 CGM.
The t:slim X2 insulin pump is compatible with the FreeStyle Libre 2 Plus sensor — giving Abbott users access to the life-changing benefits of automated insulin delivery.
The t:slim X2 insulin pump is also compatible with the Dexcom G7 CGM sensor and the Dexcom G6 continuous glucose monitor.
Learn more about Tandem Diabetes Care and how Tandem AID systems integrate with multiple CGM sensors.
Unless otherwise noted, all medical information was provided by Leah Fuller McLane, PharmD, BC-ADM, CDCES, and Amy Rich, Medical Science Liaison of Tandem Diabetes Care, Inc, and reviewed by Jordan Pinsker, MD, Chief Medical Officer with Tandem Diabetes Care.
Responsible Use of Control-IQ Technology: Control-IQ technology does not prevent all highs and lows. You must still bolus for meals and actively manage your diabetes. Please visit tandemdiabetes.com/responsible-use for more information.
References:
1. Nørgaard K, Ranjan AG, Laugesen C, et al. Glucose Monitoring Metrics in Individuals with Type 1 Diabetes Using Different Treatment Modalities: A Real-World Observational Study. Diabetes Care. 2023;dc231137. doi: 10.2337/dc23-1137.
2. Diabetes Technology: Standards of Care in Diabetes - 2024. Diabetes Care. 2024;47(Suppl. 1):S126-S144. doi: 10.2337/dc24-S007
Important Safety Information
RX ONLY.
Indications for Use:
Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use in individuals 6 years of age and greater.
t:slim X2 insulin pump: The t:slim X2 insulin pump with interoperable technology is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient use. The pump is indicated for use with NovoLog or Humalog U-100 insulin. The pump is indicated for use in individuals 6 years of age and greater.
Control-IQ technology: Control-IQ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 6 years of age and greater. Control-IQ technology is intended for single patient use. Control-IQ technology is indicated for use with NovoLog or Humalog U-100 insulin.
Warning: Control-IQ technology should not be used by anyone under the age of 6 years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of a Tandem insulin pump and Control-IQ technology must use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The Tandem pump and the CGM transmitter and sensor must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.